In the late summer of 1987 I returned to Poland to attend an international congress on insect hormones at Szlarska-Poreba, near Wrocław, in a ski resort near the Czech border. This journey involved flying to Warsaw, meeting up with other delegates at Bron’s department and then travelling through the night by bus. On this visit I found a Poland quite different from the one Jean and I had seen only a few years before when a chink of liberalism had been introduced into Polish society by the activities of Solidarnosc. By 1987 the dead hand of the Communist regime had descended again and its evidence was palpable. Nevertheless, the meeting was a good one, attended by many important workers in the field of insect endocrinology. At the end of the meeting I returned to Warsaw to stay with Bron and Malgosia before returning to Edinburgh.
Within a week or so of my return from Poland, Jean and I were off again to Chapel Hill, this time for a whole year’s sabbatical. We were met at Raleigh-Durham airport by David Richard (now a post-doc in Larry’s lab) and Michael (who had started his Ph.D. pro-gramme with Kerry Bloom on yeast genetics). We drove to Larry and Doris’s house near Carrboro where we stayed for a while before moving into an apartment in Fidelity Court. Having brought my bike with me I could ride to the lab each day, and also explore the countryside around Chapel Hill. All in all, I covered 3,000 miles during the year in North Carolina.
In the Biology department I justified my existence by giving a total of 40 lectures on Biological Clocks as well as a graduate series on Photoperiodism. This paid me a salary to finance my sabbatical and made me a temporary faculty member, as I had been in Chapel Hill in 1983 and in Stanford in 1972. By 1987 Larry’s lab was beginning to use Drosophila melanogaster – whilst not abandoning Manduca – in order to take advantage of the enormous background of molecular genetics available in the former species. So when Larry and I discussed what to do during my sabbatical, he asked the question: does D. melanogaster have a diapause?

The fruit fly, Drosophila melanogaster, a species with a shallow reproductive quiescence.
The received wisdom at that time was that D. melanogaster was ‘day length-neutral’, i.e., it was a species that had no diapause and was insensitive to seasonal changes in photoperiod. This species of fruit fly was considered to be of tropical origin moving north each summer but never surviving the winter at very high latitudes. In some text books it was stated that D. melanogaster over-wintered “in bake houses and breweries” and, in more benign climates, possibly in orchards. The truth was that very little was known about over-wintering ecology, or indeed any sort of ecology, of the geneticists’ favourite insect. However, other species of Drosophila living to the north did possess typical photoperiodic responses, with an adult or reproductive (ovarian) diapause being the most frequent response. In this form of diapause, short autumnal days cause the corpora allata to cease production of juvenile hormone(s) and the ovaries remain pre-vitellogenic (without yolk) until diapause comes to an end the following spring.
I quickly found that D. melanogaster showed a weak response resembling diapause when newly emerged female flies were exposed to short days at temperatures below about 14 °C. It was fortuitous that the initial experiments were conducted with a strain of D. melanogaster known as Canton-S, first isolated from an apparently ‘wild’ population of flies near Canton, Ohio, in the 1920s. Since that time the strain had gone through countless generations (probably mainly at 25 °C, and often under unknown or dubious lighting conditions) but apparently (and remarkably) maintaining this response intact. In contrast, another widely used strain, Oregon-R, showed no such response presumably because the climate in that Pacific state was so much more benign. As expected, the response was also absent in a tropical (West African) strain from Dahomey. Later, I had high hopes for a strain isolated in Brighton, but the complete absence of a phenomenon resembling diapause in these flies suggested that they also must have had a tropical origin, perhaps getting off a banana boat from the West Indies!
At 12 °C I found that flies exposed to photoperiods shorter that about 14 hours of light per day showed a much lower incidence of yolk deposition in the ovaries that those exposed to longer day lengths, in excess of 16 hours. The short-day response continued for six to seven weeks at 12 °C under a photoperiod of LD 10:14 but could be terminated rapidly by a transfer to a higher temperature, to longer days, or by the topical application of juvenile hormone (JH).
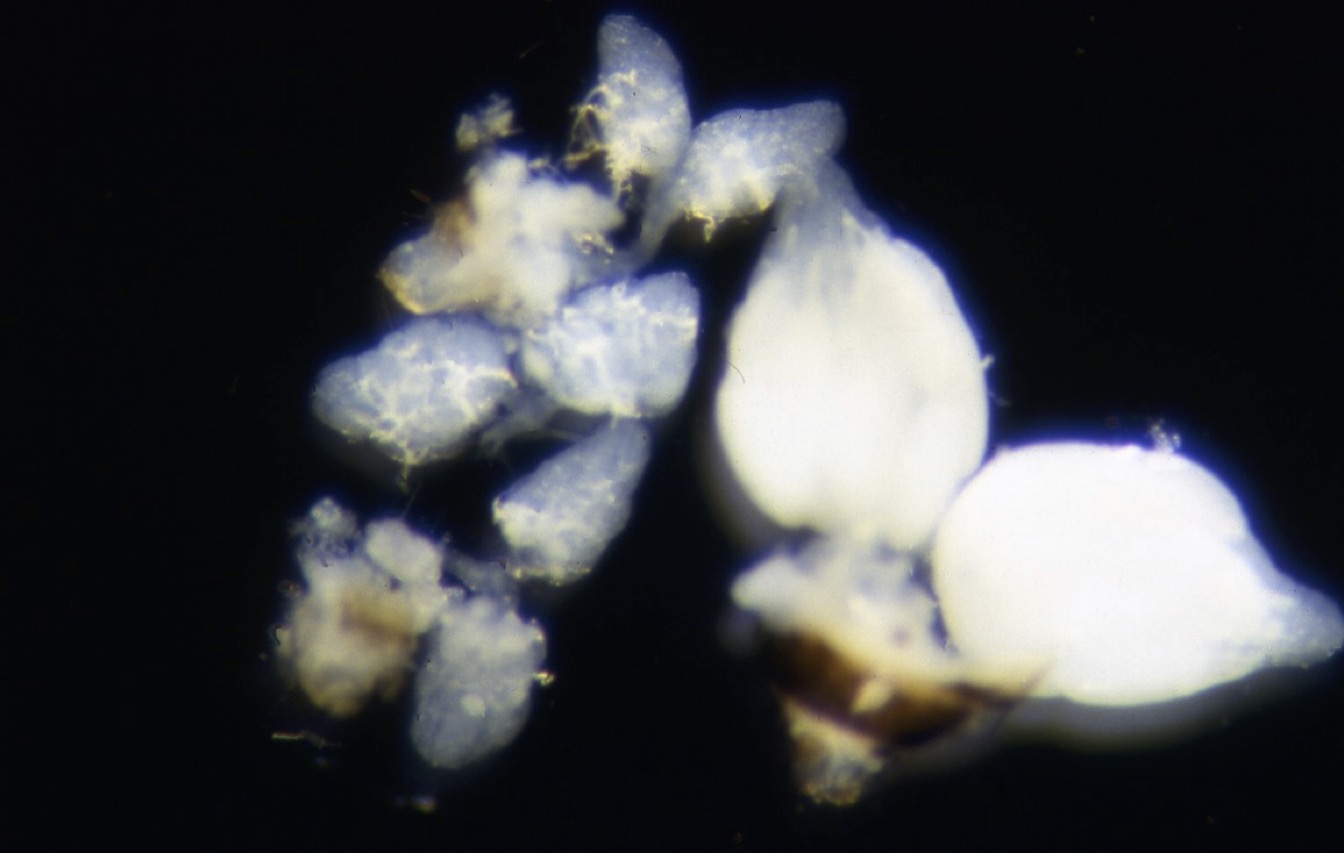
Ovaries of D. melanogaster. Left: three pairs of ovaries from flies under short days with little yolk; right: ovaries from a fly of the same age under long days with abundant yolk.
David Richard and I then collaborated on a project to determine the endocrine basis of this response in D. melanogaster (Saunders, Richard, Applebaum, Ma and Gilbert, 1990, in Gen. Comp. Endocrinol.). By using an enzyme-linked immunosorbent assay (ELISA) we were able to show that short-day females continued to synthesis yolk proteins but stored them in the haemolymph rather than transport them to the ovary. We then determined the in vitro synthesis of natural juvenile hormones (JH III and JHB3) by single excised corpora allata and showed that glands from long-day flies synthesised JH at a rate four times higher than those from short-days. It was concluded that the short-day effect resulted from a ‘block’ to the JH-stimulated uptake of yolk proteins from the haemolymph, caused by a reduction in the rate of JH synthesis by the corpus allatum. Most probably, regulation of the corpus allatum was mediated by day length effects acting via the brain.
Coupled with the fact that this short-day arrest was only evident in a proportion of the females, the condition was regarded as ‘shallow’, probably the product of natural selection following a fairly recent migration northwards from lower latitudes. By studying the weather records of various cities in the United States I found that 12 °C was the average October temperature for Canton, Ohio. I therefore considered that the phenomenon was a ‘natural’ one offering considerable selective advantages. In the autumn, as temperatures fell and days shortened, more and more flies would enter a period of reproductive arrest which delayed their reproduction until things warmed up in the spring when egg laying could resume.
The discovery of a condition resembling reproductive diapause in D. melanogaster seemed to offer new avenues of research using the powerful tools afforded by molecular genetics in what was the best-known molecular ‘model’. Also in Larry’s lab at that time was Vince Henrich, recently arrived from Jeff Hall’s group at Brandeis University in Boston. Vince had brought with him strains of flies carrying mutations of the period gene which Ron Konopka and Seymour Benzer had engineered at Caltec in the early 1970s using chemical mutagenesis. Three mutant alleles of period were known at that time: perS with a shorter than average circadian period (about 19 hours), perL with a longer than average period (about 28 to 29 hours) and per0 which apparently lacked normal circadian rhythms of either adult locomotory behaviour or pupal eclosion. Since my earlier work on photoperiodism had strongly indicated a role (and possibly a causal role) for the circadian system in photoperiodic time measurement, it was clearly important to see what effect, if any, the period mutation had on the response in Drosophila. An added bonus was that the Canton-S wild type strain used for the work described above was also the same as that used by Konopka is isolating his period mutants.
The first experiments conducted long these lines was to compare the responses of wild type Canton-S females with those of circadianly arrhythmic per0 and with a balanced (‘double deletion’) stock carrying two overlapping deletions of the per locus. Results (published by Saunders, Henrich and Gilbert, 1989, in Proc. Natl. Acad. Sci. USA) showed that all three per0 mutations studied (per01, per02 and per03) and the ‘double deletion’ flies seemed to show similar responses but with an apparent critical day length a few hours shorter than Canton-S. Our initial conclusion was that the period locus was not involved in the phenomenon.
The second approach was to determine the responses of perS and perL flies in comparison to wild type. Since these strains have a shorter than average and longer than average circadian period, respectively, one hypothesis might be that the former would show a longer critical day length and the latter a shorter one. In any case, since circadian rhythms were thought to provide the ‘clockwork’ for photoperiodism, genetic alteration of circadian period ought to have some effect on photoperiodic time measurement (PPTM). However, when this experiment was performed perS and perL were found to have the same critical day length as wild type Canton-S flies. It was concluded (J. Biological Rhythms 1990) that the period gene was not causally involved in seasonal timing. These results were thus in apparent agreement to those for per0 and ‘double deleted’ flies, reported above. Exposure of wild type and period mutant flies to Nanda-Hamner experiments then provided, at best, very weak evidence for a circadian basis for PPTM: the best conclusion was that if circadian rhythms were involved, they were probably strongly damped.
When it appeared that period had – or seemed to have – little effect on the photoperiodic induction of diapause in D. melanogaster, I phoned Ron Konopka who I had not met for ten years and was living by this time up in Maine. He almost immediately drove all the way down to North Carolina, ostensibly to see the work being carried out, but also to avail himself of the opportunity to collect butterflies in the Duke Forest!
At this juncture it became apparent that a serious anomaly was evident in our understanding of insect photoperiodism. On the one hand, formal experimentation and mathematical modelling strongly suggested that photoperiodic time measurement was a function of the insect circadian system, with the hourglass ‘alternative’ merely an expression of strongly damped oscillators. On the other hand, experiments with Drosophila melanogaster suggested that the period locus, one of the central circadian ‘clock’ genes, was not causally involved in the phenomenon. Resolution of this problem was not forthcoming for several more years, so I will delay this part of the account.
In the spring of 1988 Jean and I drove down to the Isle of Palms near Charleston, South Carolina, to attend the inaugural meeting of the Society for Research in Biological Rhythms (SRBR) where we met a number of old friends. Niall Kenny, my research student, and Marlies Vaz Nunes, then working in Edinburgh on a NATO fellowship, also attended that meeting, and we later dove back to Chapel Hill together.
Toward the end of my sabbatical in Chapel Hill we had to move out of our apartment in Fidelity Court to make way for the next occupants. Since we still had about three weeks to go before our departure, we were initially rather at a loss what to do. It was at this point, however, that Jean had another of her bright ideas: why not go on a lecture tour? In fact, Jean came to refer to this three-week trip as ‘her lecture tour’, even though it was me who did the lecturing! So we hired a car and set off on a 2,500 mile round trip visiting various friends and scientists, with me earning my keep by ‘singing for my supper’.

Flower sellers in Charleston, South Carolina
We initially drove west from Chapel Hill through North Carolina and across the Great Smoky Mountains into Tennessee, keeping off the freeways as much as possible in order to see the countryside and to sample rural American life. In Tennessee we avoided the dubious pleasures of ‘Dollywood’ and continued on to Knoxville where we checked into a motel. That evening we decided to go out for a meal rather than eat within the hotel. This turned out to be a mistake, however, because we found it very difficult to get back to our motel, and eventually had to cross about four lanes of traffic on a busy highway to make our exit! The next day we continued on to Nashville to see Terry Page and Carl Johnson at Vanderbilt University, and for me to give the first of my talks on ‘our’ lecture tour.
The journey continued northward from Nashville to Bowling Green, Kentucky, and a visit to the Mammoth Cave National Park. Then we continued on to Owensboro, through Evansville Indiana, and into Illinois where we headed north to Urbana. Keeping clear of Chicago, we arrived in De Kalb to stay with M.F. Bowen and her husband James, and for me to give the second of my talks, this time to a large audience of about 700 people in a special University lecture. We found De Kalb to be a typical mid-west town, famous mainly as the world’s barbed wire capital! After this we carefully skirted the southern suburbs of Chicago, crossed Indiana, and entered Ohio on the way to Columbus. Here we were hosted by Dave Denlinger and his wife and gave the third of my lectures. Entering Dave’s department brought back memories of my earlier work: the strong and characteristic smell of Sarcophaga! After leaving Columbus we made the long haul across Pennsylvania, stopping only to stay one night on the road and to visit the site of the battle at Gettysburg. We then continued on through Amish country to Wilmington, Delaware to stay with Ralph Quattrano and his wife; I also went to the University of Delaware campus to see Steve Skopik who was one of Colin Pittendrigh’s students.
The following day we left early to make the journey to Charlottesville, Virginia. This went fine until we hit the rush hour on the Washington D.C. beltway! After a few exciting experiences with the heavy traffic we eventually found our road into Virginia and made our way safely to Charlottesville. We stayed with Mike Menaker and his wife Shirley, and I gave yet another lecture in the Biology department. Finally we drove home to Chapel Hill by a rural route through Lynchburg and Roxboro to Durham. After a few more days we were ready to fly home.