After my return from Chapel Hill in 1983 I obtained a grant from the Science and Engineering Research Council to investigate the endocrine basis of pupal diapause in Sarcophaga argyrostoma, using techniques that I had acquired in Larry’s lab. The grant contained funds for a Research Assistant so an advertisement was placed in Nature.
One of the applicants was David Richard who had just completed his M.Sc. in Dundee at Ninewells Hospital and was an ideal candidate with knowledge of relevant techniques such as radioimmunoassay (RIA) and high performance liquid chromatography (HPLC). He was taken on as Research Assistant and signed up as a Ph.D. student.
At this stage it is necessary to outline briefly what is known about the hormonal control of moulting, metamorphosis and diapause in insects. About midway between moults, the brain secretes a neurohormone, prothoracotropic hormone (PTTH), which activates the prothoracic glands to produce the so-called moulting hormone(s) or ecdysone(s). The action of these steroid hormones (collectively called ecdysteroids) is on the peripheral tissues initiating a sequence of events leading to the development of the next stage in the life cycle. In the presence of juvenile hormone (secreted by the corpus allatum) a moult proceeds from one larval stage to the next. However, at the end of larval development the titre of JH falls and the larva moults first to a pupa, and then at the next moult, to an adult insect. Normal moulting and metamorphosis are thereby controlled by three interacting hormones, PTTH, ecdysteroids and JH. When larval or pupal diapause occurs the brain ceases to secrete PTTH, the prothoracic glands produce a very low titre of ecdysteroids, and the insect fails to moult or develop further, until the whole process is put into reverse. The ‘pivotal’ event in diapause induction is thought to be the blocking of PTTH secretion under environmental short days. In ‘higher’ flies such as Sarcophaga or Calliphora it should be noted that the prothoracic glands and the corpus allatum are both contained within a special structure, the ring gland, situated behind the brain.
Working with Sarcophaga, David Richard and I took haemolymph (blood) samples from short-day (diapause destined) and long-day (nondiapause destined) larvae and pupae every four hours, night and day, for about two weeks, and these samples were assessed for their titre of ecdysteroids (moulting hormones) by RIA. This experiment provided one of the most complete records of its kind. It showed marked peaks of ecdysteroids just before each of the larval-larval moults, and another large rise associated with the formation of the puparium (pupariation) and subsequent pupation. Following pupariation, the nondiapause (long-day) group then showed a sustained peak of ecdysteroids associated with the development of the adult fly, whereas in the diapause (short-day) line the titre of ecdysteroids fell to very low levels as a result of the failure of the brain-prothoracic gland axis to release ecdysone. Using in vitro techniques David went on to show that isolated ring glands (containing the prothoracic glands) could be stimulated to produce ecdysteroids with brain extracts containing the neuropeptide PTTH. However, ring glands from diapause-destined (short-day) pupae rapidly lost competence to respond to PTTH as they entered diapause, becoming completely refractory after 3 to 4 days. These experiments showed that pupal diapause in Sarcophaga - like that in Manduca - was regulated by a two-fold mechanism. Primary control was by the brain in which PTTH secretion was ‘blocked’ by short-day photoperiod. However, in diapausing pupae, the ring glands also become refractory to any PTTH stimulation, thus providing a two-tier, belt-and-braces control over ecdysteroid synthesis.
David and I reported these findings at several meetings including the 1985 Ecdysone workshop held that year in Edinburgh, and the 1986 International Conference on Insect Neurochemistry and Neurophysiology held at the University of Maryland. For the latter conference Jean and I flew out to Baltimore and met again with all our friends and colleagues from Chapel Hill. After the meeting we drove down to Chapel Hill with Noelle Granger - in a violent electrical storm – to stay with Larry and Doris for a while. It was during this stay that we became very close to the Gilberts, with Jean and Doris becoming best friends. Arrangements were also made for David Richard to become a post-doctoral Research Assistant in Larry’s lab, and for our second son Michael, who had completed degrees at Christ’s College Cambridge, and at Sussex, to work towards his Ph.D. at UNC. This led up to my second sabbatical at Chapel Hill in 1987 when I would work in close association with David and see much more of Michael.

At Chapel Hill with Larry and Doris Gilbert, 1986
In the early 1980s I myself performed fewer and fewer experiments on Sarcophaga, mainly because I had become increasingly allergic to this species of fly. It seemed to be the fly’s blood or haemolymph that contained the offending antigen, and the allergic reaction affected both my eyes and respiration, often after a significant time delay. For this reason I looked around for an alternative, and eventually transferred my attention to the common bluebottle or blow fly, Calliphora vicina, which produced no such deleterious affects on my physiology. Calliphora was another large fly with an endocrine system similar to that in Sarcophaga. Unlike the latter, however, it possessed a rather shallow larval diapause induced, as in Nasonia, by maternally operating photoperiods. Thus, adult female flies exposed to long day lengths during the summer months produced progeny that underwent continuous nondiapause development to the next generation, whereas those exposed to the shortening days of autumn produced progeny that entered diapause as mature, third instar, larvae in their post-feeding or ‘wandering’ stage. Whether short-day larvae actually went into diapause, however, was determined by temperature which had to be below about 15 °C for diapause to be expressed. Maternal short days and larval temperature thus work together in diapause induction.

Calliphora vicina, a fly with a maternally induced larval diapause
My work on Calliphora showed a number of similarities to that with the other species I had studied. By collecting eggs daily from short-day exposed flies it was shown that the incidence of diapause among the larvae that developed from them rose with an increasing number of short-day cycles experienced. Moreover, as in Nasonia and Sarcophaga, the number of such inductive cycles required was fairly constant over a wide range of temperatures (18 to 24 °C) indicating that this process of summation (the so-called photoperiodic ‘counter’) was temperature compensated. Experiments designed to determine whether night length ‘measurement’ was accomplished by the insect’s circadian system also – as with Nasonia and Sarcophaga – proved ‘positive’. Clearly, the circadian system in Calliphora provided the ‘clockwork’ for photoperiodic time measurement as suggested by Bünning’s original hypothesis and Pittendrigh’s ‘external coincidence’ model or, at least was very closely associated with this phenomenon.
In 1986, Bob Lewis came to Edinburgh for a few months, and we started what was to become an extremely fruitful association. He set up a system to record the circadian locomotor activity rhythms of Calliphora. Blowfly adults were placed in Petri dishes with a supply of water and sugar and their activity recorded by computer when running flies interrupted an infra-red light beam. Blow flies turned out to have very robust and persistent activity rhythms, most commonly with an average period of 22 to 23 hours. Much of this early work on locomotor rhythms was performed by a Ph.D. student, Niall Kenny. He showed, among other things, that locomotor rhythmicity presented many features in common with the induction of diapause, but also several differences. The overt system, therefore, was of limited value as the ‘hands of the photoperiodic clock’ as envisaged by Bünning for Phaseolus and proving so useful in the analysis of diapause induction in Sarcophaga. At the moment it is sufficient to note that the overt activity rhythm had a period generally much less than 24 hours and was fully self-sustained, whereas that part of the circadian system thought to be involved in photoperiodic time measurement showed an endogenous period much closer to 24 hours and appeared to damp out in continuous darkness. Clearly the two phenomena, locomotor activity and photoperiodic induction, although both circadian-based, were separate components of the insect’s circadian system, probably with different input pathways, different cellular locations of the ‘clock’ within the brain, and different outputs.

Activity rhythm of an adult female Calliphora showing its free-running circadian rhythm
Bob Lewis and I addressed these differences and complexities using his mathematical model based on a feedback control systems approach, assuming that the circadian oscillator(s) regulating photoperiodic induction were damping, even in darkness, whereas those regulating locomotor activity were fully self-sustained. In this thinking we were following Bünning who had proposed such behaviour as early as 1936 when presenting his original model for plant photoperiodism.
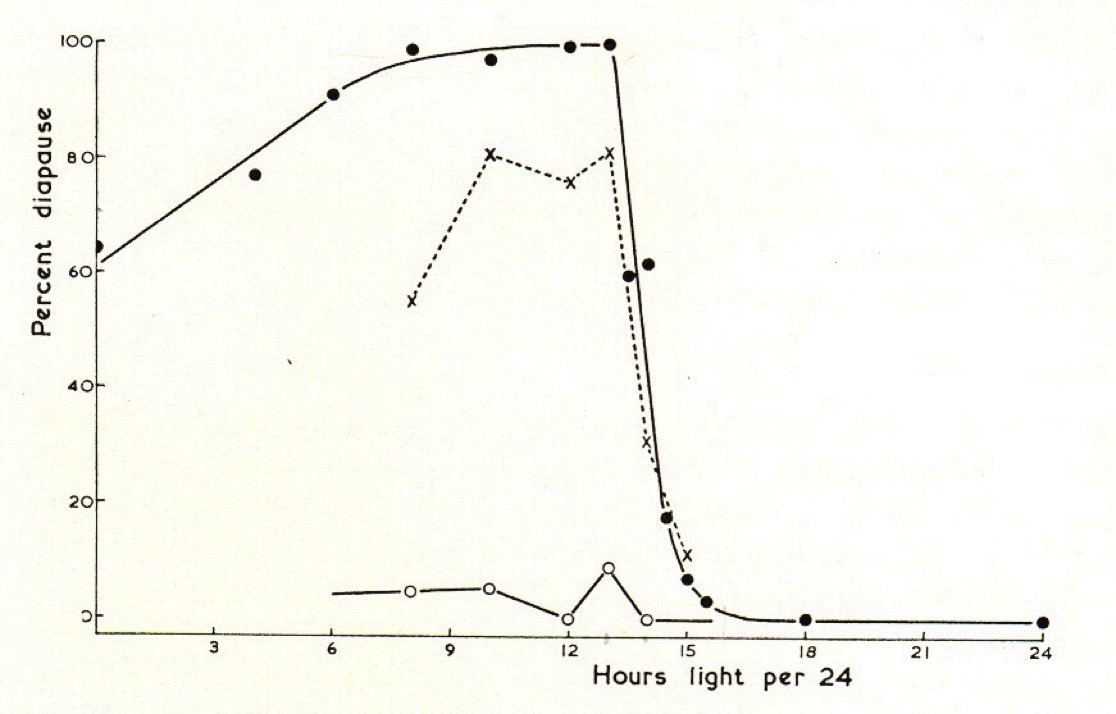
A photoperiodic response (in Sarcophaga argyrostoma) in which pupal diapause is induced by short days and low temperature
Over several decades (and continuing today) a clear schism had become apparent in the literature of insect photoperiodism, which was not so evident in similar literature concerning plants, birds, mammals and other major taxa. Whilst photoperiodic time measurement in many insects (including Sarcophaga, Nasonia and Calliphora) showed clear and compelling evidence for the involvement of circadian rhythmicity as the photoperiodic ‘clockwork’, in other species this association was less clear, and in some, such as the green vetch aphid Megoura viciae and some Lepidoptera, night length measurement appeared to be regulated by a non-circadian or non-oscillatory mechanism more akin to an ‘hourglass’. Despite many close similarities between the two apparently different types of timer, both were supported by strong and persuasive data - and vociferous support. It occurred to Bob and me, however, that the real difference between circadian and ‘hourglass’ photoperiodic timing – following Bünning - was that the latter involved an oscillatory system that was rapidly damping in extended periods of darkness. The two types of night length measurement, circadian and ‘hourglass’, were therefore essentially the same.
In 1987 we published three related papers expanding this idea in the Journal of Theoretical Biology. This work provided a theoretical model for the photoperiodic mechanism primarily in Sarcophaga argyrostoma, but more generally applicable to a wider range on insects. The model as applied to photoperiodic timing plots an oscillation of a theoretical product (chemical ‘c’) in relation to a threshold value, with the components of the system generating this oscillation being simple concepts readily identifiable in a biochemical system. These include the synthesis rate of ‘c’ (SR); its loss or degradation; the time delay (TD) between synthesis and loss; and the destruction of ‘c’ by light. The period of the free-running oscillation is to a large extent controlled by the time delay, the longer the value of TD the longer the period of the oscillation. Light reduces ‘c’ in a fashion proportional to its intensity (and/or duration). If light ‘comes on’ during the upswing of the oscillation (equivalent to the early subjective night) it reduces ‘c’ toward zero and causes a phase delay of the oscillation; if it comes on during the downswing it similarly causes a phase advance. Regulation of diapause induction was assumed to occur according to the principles of external coincidence. Thus, a photoinducible phase was thought to occur late in the subjective night. If this phase was illuminated nondiapause development occurred, but if it fell in the dark it led to diapause.
The most important aspect of the model was that of the synthesis rate (SR) of product ‘c’. If the value of SR was high the oscillation was fully self-sustained and remained above threshold. If SR was set at a low value, however, the amplitude of the oscillation slowly declined, even in darkness, until it fell below threshold and discrimination between long and short nights ceased. Most importantly, if SR was set at a very low level the oscillation damped out after a very few cycles, or perhaps even more abruptly, thus presenting the properties of a non-circadian night length timer or ‘hourglass’.
Systematic variations of the components of the model were found to simulate a wide range of different photoperiodic response curves, closely mimicking those found in ‘real’ insects. The characteristic fall in diapause incidence under very short photophases and in darkness was simulated by setting synthesis rate (SR) at very low levels, leading to an oscillation which progressively damped out unless boosted by the ‘strong’ light of longer photophases. More importantly, the model simulated a wide range of ‘positive’ and ‘negative’ experiments of the Nanda-Hamner and Bünsow type, strongly suggesting that typical hourglass-like responses could be explained in terms of a severely damped circadian oscillator – but a circadian oscillator nonetheless. Of course, modelling of this sort does not ‘prove’ such an association, but it does present its possibilities.