At this time I should outline what we know, or think we know, about insect photoperiodism, a phenomenon allowing an insect to ‘predict’ forthcoming adverse conditions (i.e. winter) by measuring daylength. I have worked on photoperiodism in five species of insects from three of the major insect orders: Nasonia vitripennis (Hymenoptera), Sarcophaga argyrostoma, Calliphora vicina and Drosophila melanogaster (Diptera) and Pyrrhocoris apterus (Heteroptera). Of these species, Nasonia and Calliphora have a larval diapause, Sarcophaga a pupal diapause, and Pyrrhocoris an adult or ovarian diapause. In D. melanogaster the response to photoperiod is doubtful, more akin to a direct response to low temperature (quiescence). In P. apterus the photoperiodic ‘sensitive period’ immediately precedes the diapause, either in an earlier nymphal instar as in Pyrrhocoris. In S. argyrostoma the sensitive period occurs in the intra-uterine embryo, and in C. vicina and N. vitripennis it occurs in the maternal generation.
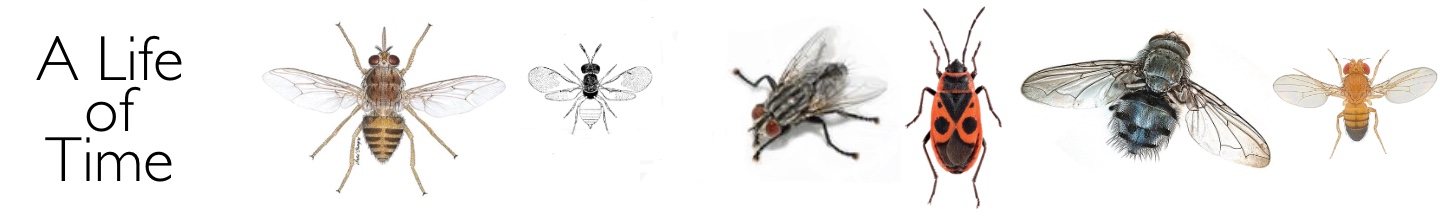
It is during the sensitive period that photoperiodic time measurement takes place. In Nasonia, Sarcophaga and Calliphora, exotic light cycles used in experiments based on the phenomenon of circadian entrainment have shown that time measurement in these insects is based, somehow, on the circadian system. The most widely used of these protocols is the so-called Nanda-Hamner experiment in which organisms are exposed to exotic light cycles consisting of a ‘short’ photophase (e.g. 12 h) linked to ever increasing numbers of dark hours, giving overall periods up to 72 h or more. In the three insects mentioned above, phases of high diapause incidence alternate with phases of low diapause with a periodicity close to 24 h (i.e. at circadian intervals). These, and other complex experiments, will not be discussed here in detail, but the results have been described by me in two recent book chapters: (1) Chapter 15, Photoperiodism in Insects and Other Animals in Photobiology: The Science of Life and Light, Lars Olof Bjorn ed. (2008) and (2) Chapter 3, Photoperiodism in Insects: The Migration and Diapause Responses, in Photoperiodism: Seasonal Time Measurement, R.J. Nelson, D.L. Denlinger & D. Somers eds. (2009).
A Nanda-Hamner response for the blow fly Calliphora vicina showing peaks of diapause incidence at circadian intervals
In the other two species, Pyrrhocoris apterus and Drosophila melanogaster, the results were somewhat equivocal with Nanda-Hamner experiments being ‘negative’ (i.e. without peaks and troughs of diapause incidence) or very weak. In Pyrrhocoris, another difficulty was observed: when the durations of the photophase (light, L) and that of the scotophase (darkness, D) were varied independently of each other, maximal induction of diapause was observed with a narrower range of L than of D. This suggested that the duration of the light was ‘more important’ than the duration of darkness, further suggesting that the duration of the photophase was the component measured by the photoperiodic clock, not the scotophase as in other insects. However, the light intensity used in these experiments (about 48 lux) was probably too low for a diurnally active insect normally found in sites exposed to very high levels of illumination. In hindsight, use of much brighter light may have revealed closer similarities with the other species examined.
The common fruit fly Drosophila melanogaster has very weakly developed diapause and photoperiodic responses. It is a species of recent tropical origin that has invaded more northern areas since the last ice age. Until recently it was thought to be devoid of a true diapause and to be photoperiodically ‘neutral’, passing the winter as a commensal species close to human habitation or “in bake-houses and breweries”. However, in 1987 during my second sabbatical at Chapel Hill, Larry Gilbert and I decided to examine possible photoperiodic responses of this insect, especially in strains from the northernmost parts of its range. Our decision was, of course, driven by the fact that D. melanogaster was - at the time - the only serious ‘genetic’ model insect of choice, with its fully characterised genome and several known ‘clock’ mutants. Thus the prospect of genetic and molecular analysis of insect photoperiodism seemed to be on offer.
Our initial investigation suggested that females of the Canton-S strain entered a weak or shallow ovarian diapause in response to short daylength. This response was most marked at the relatively low temperature of 12 °C; above that temperature most insects became nondiapause and produced a steady stream of eggs. In hindsight, this temperature was considered appropriate because reference to Whitaker’s almanac revealed that the average October temperature at Canton, Ohio, where the strain was isolated (in 1920), was indeed 12 °C! Other strains of D. melanogaster from more equable climates - such as Oregon-R (from Roseburg, Oregon) - and from tropical regions, were generally without such a response. The apparent diapause induced by short days in Canton-S was found to last for about 6 weeks at 12 °C, but could easily be terminated by an upshift in the temperature, a transfer to long days, or by topical application of natural or synthetic juvenile hormone (JH). The corpora allata of short-day, diapause induced, females were also found to have a reduced in vitro production of JH. The temporary arrest of egg development thus bore some of the hallmarks of a ‘true’ ovarian diapause, as seen in many other species of Drosophila from northerly latitudes, although the response to photoperiod was very weak, suggesting that it was indeed a quiescence rather than a ‘true’ diapause.
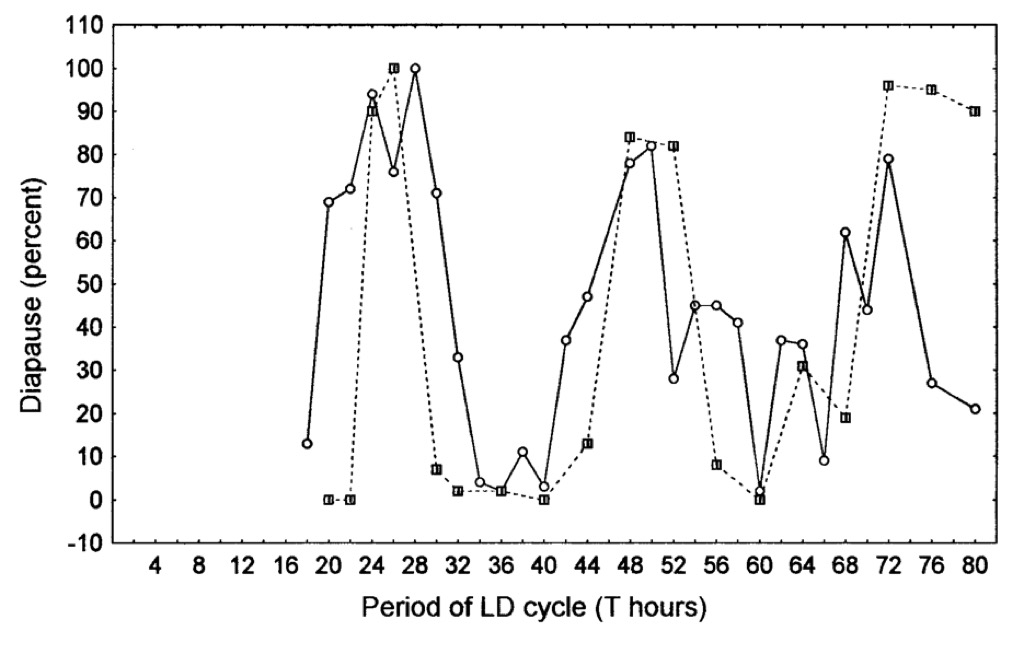
After the initial characterisation of the apparent ‘diapause-like’ phenomenon in D. melanogaster, attention was directed toward the so-called circadian ‘clock’ mutants, in an attempt to unravel their possible circadian involvement. In 1987 the only characterised ‘clock’ mutant of Drosophila was period, isolated in the early 1970s by Ron Konopka and Seymour Benzer in three allelic forms, a short period (perS about 19 h), a long period (perL about 28 h) and an apparently arrhythmic mutant (per0). Results of photoperiodic investigations using these mutant strains have already been described (see 1985-95) and will not be repeated here in detail. In short, however, it was found - unexpectantly at the time - that both perS and perL showed the same critical daylength, and that per0 and a double deletion of per (per-) both showed an apparent critical daylength, but one that was 3 h (per0) or 5 h (per-) shorter than wild type. These results did not support any obvious role for period in the Drosophila photoperiodic clock, and that was the conclusion presented at the time in published work (J. Biological Rhythms, 1990). In hindsight this conclusion may not be valid, and period might play a part in the photoperiodic clock. Evidence to support this view comes from several sources.
Photoperiodic responses of Drosophila melanogaster at 12 °C. A – perS and perL2 compared with wild type Canton-S; B – per0 and per- compared with Canton-S wild type.
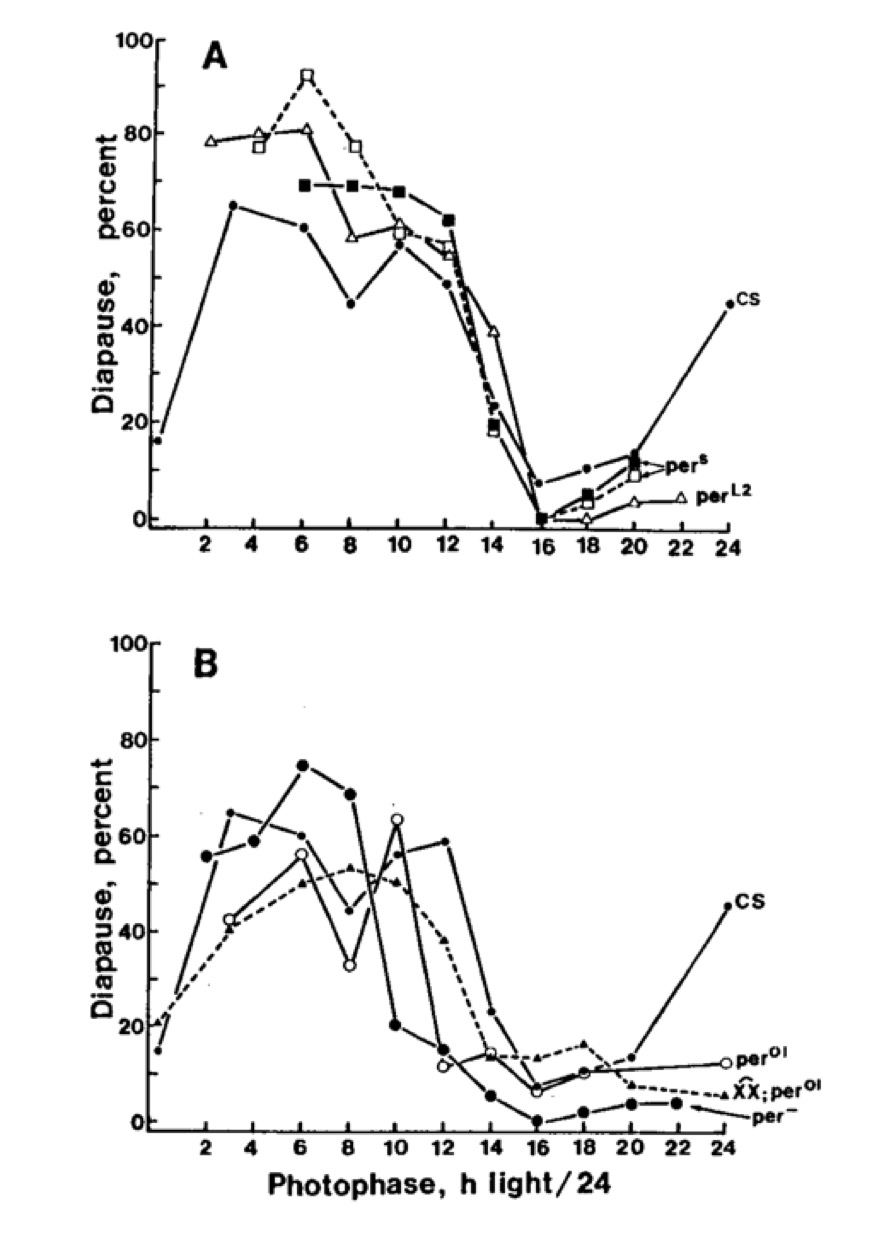
The lack of any difference between the critical day lengths of perS and perL2 may be explained by the use of the low experimental temperature (12 °C), close to the lower developmental threshold for D. melanogaster. In the same year that we published our initial observations on the photoperiodic responses of period mutant flies (1989), Ron Konopka, Colin Pittendrigh and Dominic Orr (J. Neurogenetics 6: 1-10) showed that the period of perL shortened, but that of perS lengthened, as the temperature fell to about 15 °C. Although the endogenous periods of these mutant strains could not be determined at the even lower temperature of 12 °C because of the behavioural inactivity of the flies, extrapolation of the data to this temperature suggests that the periods of perS and perL2 become might become indistinguishable, thereby eliminating any possible differences in critical daylength.
The effect of temperature on circadian period in D. melanogaster (from Konopka, Pittendrigh & Orr, 1989). Extrapolation of these data to lower temperature suggests that periods may become indistinguishable at 12 °C
The changes in critical daylength for per0 and per- flies may be explained thus: In the former the circadian photoperiodic component was reduced because of the truncated period protein, and in the latter it was entirely eliminated. The rise in diapause incidence observed under short days in both strains was then due to the direct diapause-inducing effects of low temperature. At 12 °C, however carefully one tries to regulate the temperature within the experimental cabinets, flies exposed to a short daylength will receive less radiant energy than those exposed to long days, and the additional heat absorbed by the tissues of the flies’ bodies may well have altered the diapause response in a manner distinct from a true photoperiodic response. These new interpretations of older data mean that the initial conclusion – that the period gene and its protein are not causally involved in photoperiodism – may be invalid. In future investigations one should not rule out an important role for this ‘clock’ gene.
At this point the investigation could go no further. D. melanogaster was, and remains, one of the best molecular systems for the analysis of many aspects of insect physiology, but its diapause and photoperiodic responses are too weak and variable to provide a good model for these phenomena. In short, a new genetic model was needed. Other species, with a more robust diapause, but lacking the necessary genetic background have been adopted by other research groups. These include the drosophilid Chymomyza costata, the linden bug, Pyrrhocoris apterus, and the flesh fly, Sarcophaga crassipalpis. Some progress has been made using these insects, but what is needed is a species with a robust diapause and photoperiodic response, strong evidence for the circadian involvement in time measurement, and a good genetic background. Such a species is the parasitic wasp Nasonia vitripennis whose genome has recently been unravelled in a project led by Jack Werren at the University of Rochester, N.Y.

Lecturing at Groningen, 2007
In 2007 I was invited by colleagues at the University of Groningen to lecture on insect photoperiodism. Of course, I was talking about work that I had done some 30 or 40 years ago - which only goes to show that everything comes to those that wait! I gave two lectures: one general talk on insect photoperiodism to an open audience, and one more specialised talk on Nasonia vitripennis to the Chronobiology and Evolutionary Genetics groups working on that insect in Groningen. My invitation arose from the fact that these groups were contemplating a new investigation of insect photoperiodism, using the parasitic wasp, now that its genome had been characterised.

With members of the Nasonia group in Groningen. Left: Rinaldo Bertossa; right: Leo Beukeboom
After my visit to Groningen, an application was made to the Dutch research foundation for a grant to investigate insect photoperiodism in Nasonia vitripennis. Rinaldo Bertossa became the postdoctoral fellow employed to perform the molecular genetics, and I became one of the “advisors”. We met again in Edinburgh in June 2008 at the “Nasonia08” meeting where I gave another talk on this insect’s diapause and photoperiodic responses.
[David's memoir concludes at this point, though the headings below suggest he planned to include reports of some further meetings.]
Budapest 2007
Groningen 2009
Switzerland 2010

With Rudi Costa in Hungary, 2007