My fourth decade saw a change in research activity, from age determination in tsetse flies to a study of insect photoperiodism and the occurrence of winter dormancy or diapause. As noted above, the first of these research programmes represented the ‘past’ since the permanent changes found in the ovaries were a record of earlier reproductive events, whereas the latter represented the ‘future’ since insects were found to be able to respond to changing autumnal day length and prepare for winter. It should not be thought, however, that insects (or other organisms) can actually foresee impending winter; it is just that those individuals that have the ability to respond to shortening days, suspend their development, lay down additional fat reserves and sometimes also acquire a degree of cold tolerance are better fitted, in a Darwinian sense, for successful over-wintering and subsequent breeding the following spring. There is therefore an enormous selective advantage in being photoperiodic and entering diapause, and this facility must have evolved in many species. The change from a study of age determination to that of photoperiodism did not happen suddenly. Indeed, work on tsetse flies continued for a few more years, up to about 1971; this work will be described first, for the sake of continuity.
My method for determining the physiological age of wild-caught tsetse flies was adopted by a number of workers in various parts of Africa, notably Jim Harley at EATRO, and A Challier at the Centre Muraz in Bobo-Dioulasso, Upper Volta (now Birkina Faso). Challier made the method much more user-friendly by noting that the order of size (and hence of ovulation) in newly-emerged flies was always 4, 2, 1 and 3 when observed from the dorsal aspect and read from left to right. He called this the nombre repère (repeated number) for reasons that will soon become obvious. After the first ovulation, which leaves a relic in the inside ovariole of the right ovary (number 1 above), the follicle in the inside position of the left ovary becomes the largest and they all move up one place in the sequence, so that the nombre repère becomes 3142. After the second ovulation and with relics in both inside ovarioles, the nombre repère becomes 2431, and after the third ovulation, 1324. The whole cycle is then repeated with females showing a nombre repère of 4213 after four ovulations. After that things become somewhat uncertain because flies never show more than four relics, one in each ovariole, but the nombre repère still proceeds through 3142, 2431 and 1324. Challier was thus able to recognise eight age categories (0 to 7) and extend the method with a fair degree of accuracy to flies as old as 70 days, although his categories 4 to 7 still contained a residue of older flies not differentiated. For example, flies showing evidence of four ovulations and a nombre repère of 4213 could have produced 4, 8, 12 or even 16 eggs, but were still designated 4 + 4n. Similarly, flies showing four relics but 3142 could have produced 5, 9, 13 or even 17 eggs, and were placed in category 5 + 4n. Still older flies in categories 6 to 7 (all with a follicular relic in each ovariole, but with nombre repères of 2431 and 1324) were placed in categories 6 + 4n to 7 + 4n, and so on.
I was anxious to meet Challier, and to see Glossina palpalis, the main vector of classical human sleeping sickness, in its natural environment, so a visit to West Africa was arranged in 1966 with a grant from the University of Edinburgh’s Greig Fund. On this trip I intended to visit Challier in Bobo-Dioulasso, then go on to Congo Brazzaville and finally to the Nigerian Institute of Trypanosomiasis Research (NITR) at Kaduna, Northern Nigeria. I obtained a visa for Upper Volta at their embassy in London, but a similar application to the Congolese embassy was met by a refusal, mentioning the attempted Unilateral Declaration of Independence (UDI) by the white settlers in Southern Rhodesia, now Zimbabwe, as a reason. They suggested that I should try again at the Congolese embassy in Abidjan, Côte d’Ivoire, one of my intended stop-overs.
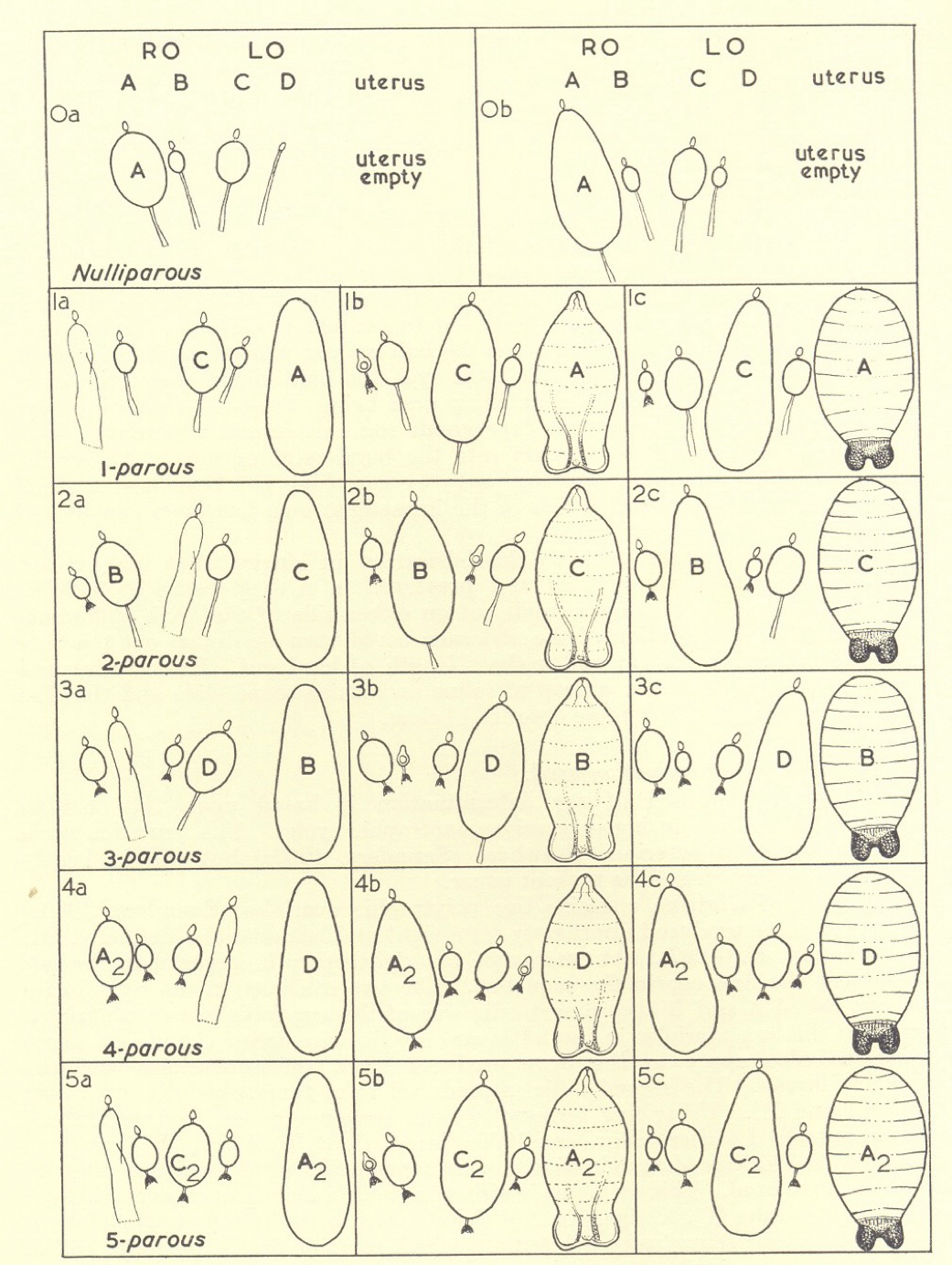
Determination of physiological age for female tsetse flies, according to the number and position of follicular relics in the four ovarioles and the stage of development of the larva in the uterus.
I set off for West Africa on an Iberian Airways flight to Madrid, and then on with touch-downs at Dakar and Conakry to Monrovia for an overnight stop. I saw nothing of Liberia since it was dark and the airport was some distance from the capital and found it somewhat strange to be in Africa yet use American dollars for settling my hotel bill. The following morning I continued to Abidjan and spent the day in a fruitless attempt to obtain a Congolese visa. This failure meant a change in plan, and I cabled NITR in Kaduna to say that I would be arriving somewhat earlier than planned and would be staying for six weeks!
The northward journey from Abidjan to Bobo-Dioulasso was by train and was scheduled to take about 24 hours. However, there was an accident on the line (I think a child was killed when trying to cross the line) and the journey took over 27 hours. When the time came for an evening meal I went along to the dining car to find it swimming with beer! On top of that there had been a mass invasion of winged termites which were drowning in the beer. All the mess was cleared up, an immaculate white tablecloth and silver cutlery appeared, and a more than passable meal was served, sufficient testament to French life in Africa! On arrival in Bobo-Dioulasso I proceeded to the Centre Muraz and stayed in their guest apartments.
Challier took me out to an area of forest to collect tsetse flies. We parked the vehicle in a forest glade which was dominated by a deep and fast-flowing stream, one half of which was as clear as crystal and the other half brown with mud. If this had been in ‘British Africa’, lunch would have been a wilting sandwich and a thermos flask served under a thorn bush. But here the French tradition was again strong: Challier produced a folding table, linen and cutlery from the back of the car and an excellent picnic, apologising that, in Africa, the vin rouge had to be served chilled! C’est la vie!

A Glossina palpalis habitat in Burkina Faso
After a couple of days in Bobo-Dioulasso, I made the return journey to Abidjan by train, then flew on to Lagos via Accra, and then up to Kaduna in Northern Nigeria. Tom Leach the acting Director of NITR at that time proved to be an excellent host and made all the arrangements for my stay. It was decided that I should travel to a known tsetse area in the central part of Nigeria, near Abuja. So, armed with a boxed microscope and all the accoutrements necessary for field entomology I travelled by train from Kaduna to Minna, a town about 90 miles to the south-west. At the ticket office I was offered the choice of the Express or the Local. Not wanting to speed through a novel stretch of country, I opted for the latter. This proved to be a mistake! The train stopped at every village and halt between Kaduna and Minna and was sometimes moved into a siding to let the express pass through. At each stop an impromptu market appeared by the side of the track, allowing me to stock up on bananas and other fruit. This part of the journey took practically all day and it was well into the afternoon before I reached Minna. The onward journey was then by bus – a small, rather broken-down ‘Mammy Wagon’ type of vehicle adorned with religious slogans. When asked about the fare, the driver said, “50p for Africans, £1 for Europeans”! I paid my £1 and got to sit up at the front with the driver for the 60-mile journey to the Abuja emirate. Abuja is today the Federal capital of Nigeria, but in 1966 was a small rural town. I stayed at a Government ‘Rest House’ that comprised a group of attractive cottages. The food was excellent, with quite good curries and a memorable palm-oil chop.

My transport to Abuja
Before I started collecting flies, I had an audience with the Emir of Abuja at his palace and hired a bicycle (5 shillings for the day, but two flat tyres) for a trip around Abuja to visit outstanding rock formations close to nearby villages. I was also taken to see work being carried out on Onchocerciasis, a nematode infection sometimes causing ‘river blindness’ and transmitted by a species of Simulium, a biting black fly. As part of their work on this debilitating disease, I observed a ‘skin snip’ survey where the local population patiently lined up to have a small piece of skin excised from the hip to look for the worms as they travelled through the body. The incidence of worms was quite high in that area.
The area selected for my investigation was a G. palpalis locality near Izom. Flies were collected by hand and transported back to Abuja in glass tubes for dissection. I found that the best way of handling these flies was sitting in bed under my mosquito net. This way any escaping flies could be quickly and easily retrieved. A total of 277 flies were collected, of which 117 were females. As expected this sample contained a large number of very young, in many cases teneral, flies, probably coming to human baits for their first blood meal. The largest group, however, was of flies showing four follicular relics and therefore over about 40 days old. These flies could be further divided according to Challier’s improved method, but clearly contained much older flies that had completed five, six or more reproductive cycles. In order to make some sense of this age distribution I split up the older and indeterminate categories by extrapolation, assuming a logarithmic death rate. This demonstrated that a few flies may have lived to a quite extreme old age, producing a large number of eggs and hence larvae. The data suggested that the population of G. palpalis at Izom was fairly stationary, or perhaps slowly increasing.

My room at Abuja, where I dissected flies under my mosquito net.
After my return to Kaduna, Tom Leach and I drove to the second NITR laboratory up on the Jos plateau where most of the protozoological work was carried out. However, by that time the Biafran war had broken out and we were stopped on both legs of the journey at road blocks manned by wild-eyed youths who demanded that we open our luggage on the road to look for offensive weapons. The Biafran war then made my homeward journey to Edinburgh rather difficult because many of the Nigerian Airways planes leaving Kano for London were commandeered by the Nigerian army to ferry Northern officers up from the south of the country. But, after several days’ delay, I did make it home.
In 1966 I obtained a substantial research grant from the Welcome Trust to fund a laboratory colony of tsetse flies at the Zoology department in Edinburgh. This colony was to be modelled on the only successful laboratory colony at that time, the one at the Tropical Institute in Lisbon run by Professor J. Fraga de Azevedo and his colleague, Dr R. da Pinhão. In September I travelled to Lisbon for a few days to see how it was done. At that time, breeding tsetse flies in the laboratory was considered to be, at best, arduous and, at worst, impossible because of the difficulty of blood-feeding the flies and their very slow form of viviparous reproduction. Professor Azevedo and his colleagues had succeeded where others had failed by using the shaved sides of guinea pigs as a source of blood. Back in Edinburgh my grant covered the expenses of Alistair Mews who very ably supervised the day-to-day running of the colony, a technician and a team of two or three part-time ladies whose job was to feed the flies kept singly in small glass tubes or in small groups in gauze-covered cages. Shortly after starting the colony we moved to using the ears of lop-eared rabbits as a source of blood, as had been pioneered by T.A.M. Nash at Bristol, and the Edinburgh colony, although always small, became the world’s first completely enclosed laboratory population of G. morsitans, producing about 10,000 pupae a year.
After about 18 months of continuous expansion, however, reproduction almost stopped. Examination of the female flies revealed a high incidence of abortion and degenerate egg follicles within the ovaries. Comparison of the Edinburgh flies with Bristol-reared flies maintained under identical conditions, and with Edinburgh flies raised entirely on human blood (the palms of my hands!) showed that the effect was dietary and maternal in origin. The trouble was eventually traced to a change in the diet fed to the rabbits, a change that occurred about a month before the phenomenon first appeared. This suggested that an unidentified ‘toxicant’ was concentrated by the female parent and incorporated into larval tissues during intra-uterine development. The nature of this toxicant was never ascertained, tests for the presence of the insecticides DDT and γ BHC only showing very low levels in the offending rabbit diet. The problem was resolved by a move back to the original, uncontaminated, diet and successful reproduction soon followed. Some time later, Peter Hill, Allan Campbell and I fed flies on rabbits injected with a variety of antibiotics. Tsetse flies feed only on vertebrate blood and, in common with other insect parasites with this habit, harbour symbiotic micro-organisms in a special portion of the midgut called a mycetome. These symbionts provide the fly with essential nutrients, presumably in exchange for a safe existence within the fly. The antibiotics were shown to kill these symbionts, and the flies developed reproductive abnormalities similar to those seen in flies fed on rabbits receiving the offending diet. This work was never taken further. I can’t help feeling, however, that this episode was a lost opportunity for the potential control of tsetse populations in the field. If the cause of the reproductive abnormalities, whether a toxicant or an antibiotic in the rabbits’ food, had been identified, these materials could have been fed to grazing cattle, and even to wild game, by their incorporation in salt-licks.
I must here digress to describe a visit to Iran.