A tsetse fly (Glossina sp.) feeding.
Tsetse flies are unusual among the insects (although not unique) in reproducing by viviparous means, in a fashion superficially reminiscent of the mammals. They produce one egg at a time, at intervals of about 10 days, from just one of the ovaries. This egg passes down the ovariole and lodges in an expanded portion of the common oviduct that now acts as a uterus. Fertilisation takes place using sperm stored in paired spermathecae (tsetse flies need to mate only once in their life-time) and subsequent larval development takes place in the uterus, the developing larva feeding on a secretion, dubbed the ‘milk’, produced from paired accessory glands. At the end of the period of gestation which lasts about 10 days at 25°C the fully developed larva is expelled from the uterus, burrows into the soil and forms a puparium. Shortly after birth the egg shell and the discarded larval skins are expelled, rather like the mammalian placenta. A few hours later another egg descends into the uterus and the second pregnancy begins. Single larvae are thus produced at 10-day intervals non-stop unless the sequence is interrupted by an abortion. All this happens after a single act of insemination: a female tsetse fly has little fun but much labour!
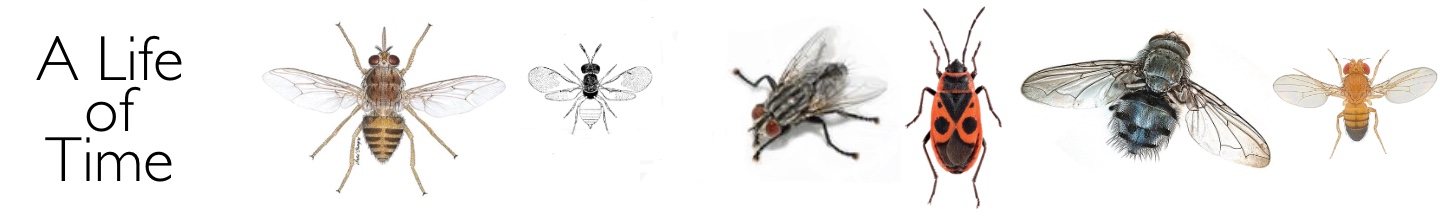
Since only one egg is produced at a time, the two ovaries are small and of different sizes. In every newly-emerged fly dissected, the right-hand ovary is the larger and produces the first egg in the sequence. The second egg, after the first pregnancy, is produced from the left ovary, and then right and left ovaries ovulate in a strict sequence.
Investigation of possible age changes in the ovaries of Glossina necessitated careful dissection. All of the textbooks of that time stated that tsetse fly ovaries contained a single ovariole, even though related insects such as the hippoboscid ked fly, Melophagus ovinus, were known to have two. As soon as I teased open the ovary of G.morsitans with my dissecting needles I found that there were in fact two ovarioles rather than one. This was the second entirely serendipitous discovery of my career – after my own personal discovery of photoperiodic diapause in Nasonia - and it made the whole venture immediately more interesting and exciting. I rushed a letter off to Nature (in those days this illustrious journal accepted reports of work ‘in progress’), and subsequently showed that other species of tsetse flies (G. palpalis, G. pallidipes and G. brevipalpis) were similarly endowed. In every newly-emerged fly examined, the largest egg (and the first to be ovulated) was in the inside position in the right ovary. The next in size was inside left, the third outside right and the fourth outside left. This immediately suggested the sequence of ovulations which I likened to a football team (although I have no love of that game!). When I followed the growth of the developing eggs in blood-fed flies through four or more cycles of ovulation and pregnancy, this is the sequence that emerged.
The ovaries of newly emerged tsetse flies, Glossina morsitans, G. pallidipes, G. austeni and G. brevipalpis.
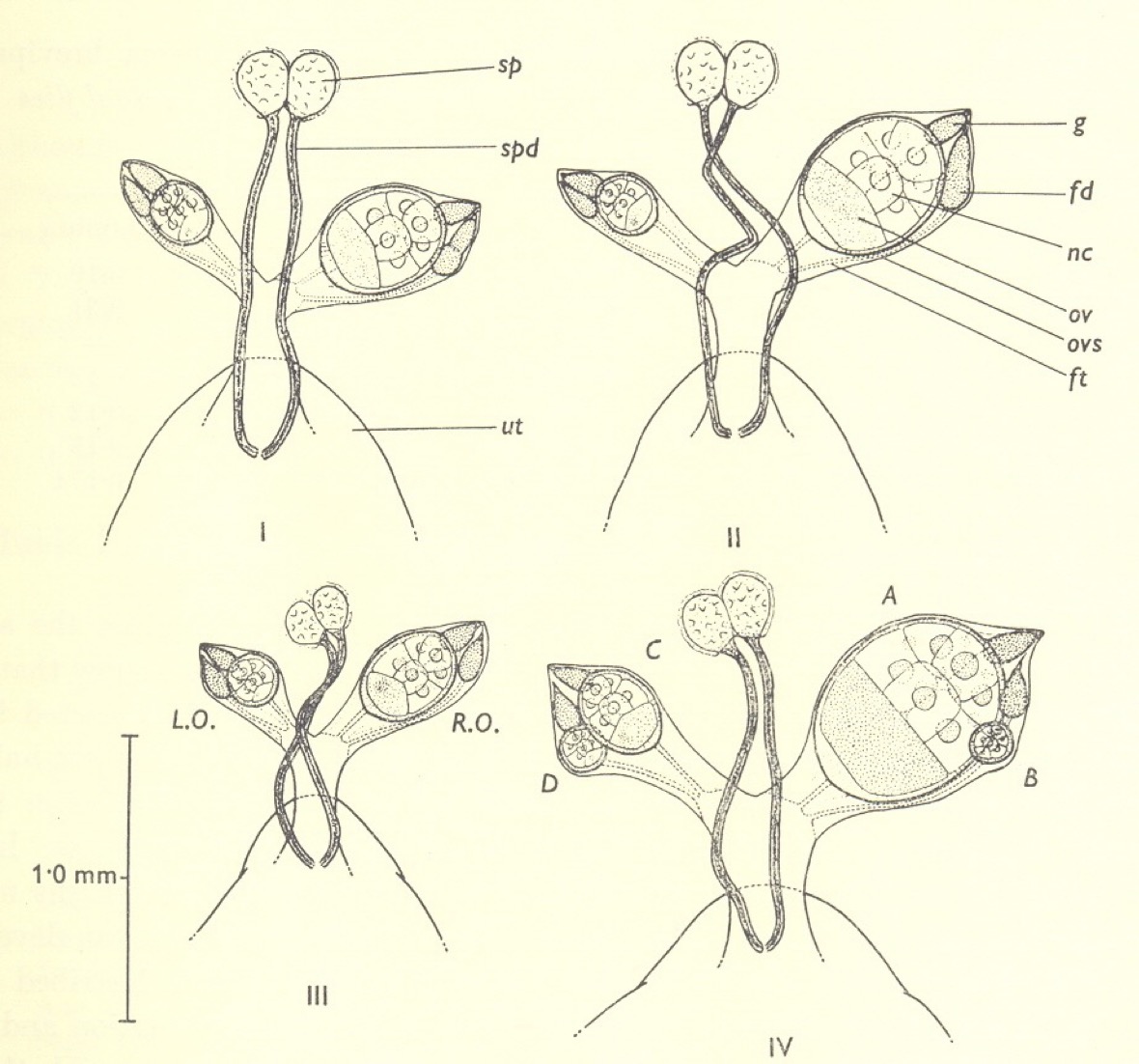
As in mosquitoes, an ovariole immediately after ovulation showed a large expanded ‘sac’ in the follicular tube containing some cellular debris. Over the next few days this sac shrunk to give a small follicular relic marking that ovariole as one that had produced an egg – the egg that was now in the uterus and would hatch into the first larva. Relics appeared in due course in the three other ovarioles as they ‘fired’ in sequence. A significant difference then emerged between tsetse flies and mosquitoes. When the ovariole in the inside right position ovulated for the second time, to produce the fifth egg in the overall sequence, two relics were not produced. It appeared that the very large egg ruptured the distal end of the follicular tube where it joined the bottom part of the ovariole and removed any remnants that might have persisted from earlier ovulations. The only exception to this was if an egg degenerated in the ovariole: in this case its relic persisted.
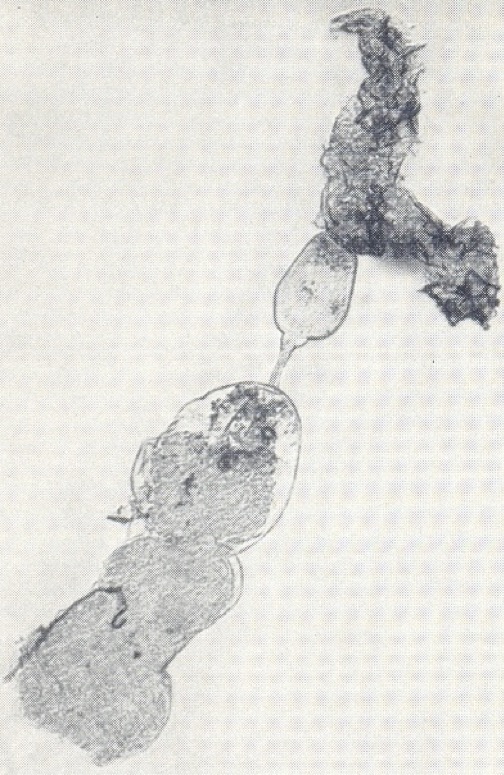
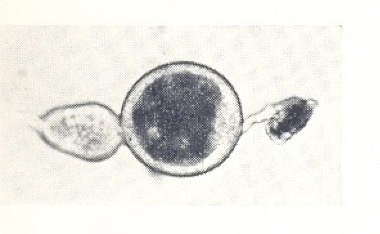
Ovarioles of G. pallidipes dissected from ovaries: above – shortly after ovulation showing an expanded ‘sac’; below – some time later showing a small dark follicular relic.
Clearly this was a serious limitation to the method as applied to tsetse flies. Since each ovariole could show evidence of only one ovulation, even if two or more had occurred, it meant that older flies that had completed four or more ovulations could not be distinguished and sub-divided. Nevertheless, since each gestation and inter-ovulation period lasted about 10 days, flies could be aged with a degree of accuracy for about the first 30 to 40 days, considerably in excess of the three weeks that the pathogenic trypanosomes take to develop to their infective stage within the flies. The other short-coming of the age determination method in tsetse flies when compared to that in mosquitoes was that males were also blood-sucking and important potential vectors, and the ovarian method, of course, was not applicable to them. So, compared to the extremely elegant technique for mosquitoes, the method as developed for Glossina had its limitations, but was still vastly superior to older methods such as those, for example, relying on the degree of wing-fray.
The method of age determination, as described, provided an accurate record of the number of ovulations and pregnancies that had occurred, and was thus a manifestation of past events. However, it was not a true ‘biological clock’, since these reproductive events are not temperature compensated, occurring at a higher rate with a rise in temperature. As such it was an estimate of physiological and not of chronological time. Nevertheless, since the effects of temperature on reproduction and on the development of trypanosomes within the fly are probably similar, and run hand in hand, the method of age determination as described offers a valid method to assess the epidemiological importance of potential vectors.
Naturally, I was now keen to apply this age determination technique to wild-caught tsetse flies in their natural habitat. During 1960, Dr Russell Lumsden, Director of the East African Trypanosomiasis Research Organization (EATRO) passed through Edinburgh (which was his home town) whilst on leave from Africa, and invited me to his laboratories in Tororo, Uganda, to test the method under field conditions. I obtained a research grant from the Colonial Office that paid for my fare to Africa and provided £50 a month for subsistence, and I stayed in Tororo for four months during 1961. At this point I must say that my long-suffering wife stayed behind in Edinburgh with two young children and encouraged me, as always, to take this opportunity. Two years later, however, we were able to make a return visit to Tororo as a family. Without Jean’s support and encouragement, I doubt whether I would have achieved half of what came my way in life.
I flew out to Entebbe on one of the ill-fated Comets via Rome and Benghazi and was met at Entebbe by a driver from EATRO for the onward journey to Tororo, close to the border with Kenya. This was my first time in Africa – indeed outside Europe – and everything seemed to be colourful and exciting. I was installed in a small bungalow on the EATRO ‘campus’ and employed a house boy called Odwor to look after me. It was only a short walk to the lab each day enlivened by the exciting local insect fauna. Often I would observe a pile of animal dung on the path which had disappeared by lunch-time after the scavenging activity of dung beetles. Butterflies and moths abounded at Tororo, and the latter were attracted to the light in the bungalow’s porch each night and were there to be closely observed the following morning.

My bungalow in Tororo, Uganda, 1961
We decided that the best way of testing the age determination method on wild flies was to perform a type of ‘release-and-recapture’ experiment. In this I was greatly helped by Jim Harley, EATRO’s resident entomologist, and a group of technicians or ‘fly boys’. The tsetse fly zone was some distance from EATRO down on the shores of Lake Victoria, and we travelled down to this area two or three times a week. We had the use of a boat (called - appropriately for the times - “The Wind of Change”) and selected an isolated island in Lake Victoria, called Namulamia, as the site for the experiment. The islands in this part of the lake are rather interesting. Some are so-called ‘spider islands’ literally covered with the webs of Nephila spiders, sometimes so densely covered that small spiders had to spin their webs within the webs of their large cousins. These spider islands seemed to act as giant nets probably gathering the swarms of insects, especially Chironomids, which flew over the lake. Other islands, such as Namulamia, were almost entirely devoid of spiders, and it was assumed that such islands would be more likely to possess resident populations of Glossina. The fly boys cut a path around the perimeter of the island and expertly sieved puparia of G. fuscipes from the soil. These puparia were taken back to EATRO and the newly emerged flies then marked with different coloured paints on the back of the thorax in such a way that recorded their date of emergence. They were then returned to Namulamia and released. Flies were then collected at intervals as they came to the group of fly boys as they scrambled around the island’s perimeter. We also took a calf out to the island to attract flies, and on one occasion a 10 foot long python, knowing that palpalis-group flies were also attracted to reptiles. As in classical release-and-recapture experiments, the recaptures were rather infrequent with only a small number of marked flies being recaptured. Of the 1,440 marked flies released (720 males and 720 females), 130 (18 %) of the males were recaptured, but only 8 (1.11%) of the less active females. These, however, were of an exact known age, and could be dissected back at the lab to ascertain their reproductive history. They ranged in age from 2 to 55 days and, upon dissection, ages estimated by the ‘ovarian’ method were found to tally exactly with known age, so validating the method to a great deal of satisfaction.

Aboard the ‘Wind of Change’, Lake Victoria

Namulamia Island, Lake Victoria, site of the release-and-recapture experiment with Glossina fuscipes.
On the mainland, I also collected G. pallidipes by three methods, by hand, on bait cattle and in Morris ‘animal’ traps (traps original designed to “look like” animals), and then dissected the females to assess their age. This experiment showed quite clearly that trap-caught females were the oldest and hand-caught the youngest, confirming what had already been known. Those flies caught by hand, coming to human baits, also contained a high proportion of newly-emerged or ‘teneral’ flies, presumably coming for their first bloodmeal. Teneral flies were virtually absent from the trap-caught samples. The very young age of flies coming to human baits, as compared to those coming to a bovine, suggests why G. pallidipes is likely to be a more efficient vector of animal (Nagana) than human (sleeping sickness) trypanosomes.
On my way back from Uganda, I made stop-overs, at no extra cost, in Khartoum, Beirut (to visit my dentist friend, Zahi,), Athens and Rome.
In 1963 I returned to Tororo for a second four month period, this time with Jean and two small children (Robert, aged 3½ and Michael, aged 2). During this visit I carried out an experiment using an 8 x 8 Latin Square design, with eight different sampling methods, rotating around eight different sites, for eight consecutive days. The eight sites selected comprised three close to the thicket edge, two in isolated clumps in open grassland, and three in the open grassland itself. The eight sampling methods included two black Zebu oxen and six modified Morris ‘animal’ traps. The numbers of G. pallidipes coming to the various attractants were recorded, the flies returned to EATRO over dry ice for dissection, and the physiological ages of the females determined using the ovarian age-grading technique. The experimental data were analysed after my return to Edinburgh. In such a pre-computer age, analysis was performed on an electro-mechanical Madas calculator, and took nearly six weeks to complete!

Collecting flies with a Morris trap, 1963
After this second trip to Uganda I wanted to combine research visits to various East African laboratories with some sightseeing. I took advantage of a lift to Nairobi, continued by local bus to Arusha in Tanganyika, and then a second bus down past Kilimanjaro to Amani to visit the East African Malaria laboratory. Here I remember impressing Mike Gillies as one of the very few visitors able to apply the age grading technique to mosquitoes: presumably my experience with tsetse flies, although they were much larger, helping with this. After a day or so in Amani, I continued on through Dar es Salaam to fly to Zanzibar for a fascinating few days. I had then intended to travel overland back through Nairobi and on through the Sudan and all the way to Cairo, but the continuing war in the south of the Sudan meant that I had to cut out this part of the trip and fly straight to Khartoum. In Khartoum I stayed with John Cloudsley-Thompson who had been appointed to the chair of Zoology at the University, and was particularly interested to see some of the University buildings erected before the war under the guidance of Jean’s Uncle Tom (Thomas Doughty) when he was Dean of Engineering at the Gordon College, as it was then. John and I went out to the site of the Battle of Omdurman and to the White Nile dam where the temperature was an impressive 47 °C. The onward journey was then by train across the desert to Wadi Halfa, then by boat down the Nile to Aswan in Egypt. In 1963 the waters of the newly constructed Lake Nasser were beginning to rise, in many cases covering invaluable sites of great antiquity. We persuaded the boat’s captain to make an unscheduled stop at Abu Simbel, however, and were able to see this unique monument before it was cut into pieces and moved, block by block, to higher ground. On board the ship I met an Australian, also going overland, and we continued together to Cairo. I also met a German student who was travelling 3rd class along with the goats and chickens. So exhausted was he after the first night, I let him use my cabin during the days whilst I slept at night! We broke our journey at Luxor where were checked into the original Winter Palace Hotel. Visits were made to the Temples of Luxor and Karnak, and across the Nile by felucca and local taxi to the Valley of the Kings, to see the burial chambers of the pharoahs Tuthankamun and Ramesses the Great. (I was not to repeat this visit for another 40 years, until returning with Jean in 2002). After Luxor we carried on to Cairo by overnight train, visiting the Cairo museum and the Pyramids, before I flew home to Edinburgh.

At the Pyramids, 1963
In 1962 there had been some changes to the Parasitology group in the Edinburgh Zoology department. Doug Kettle had returned from his two-year sabbatical in Jamaica, only to be appointed to the chair of Zoology in Nairobi. He was replaced by Allan Campbell from the nearby Moredun Institute – and I was promoted to a lectureship. Allan was a true polymath, speaking several languages including Spanish which he learnt whilst an ambulance driver for the International Brigade during the Civil War. He had an enormous knowledge of all things parasitological, and I owe him a considerable debt in imparting at least some of that knowledge to me. Together we taught Arthropod Parasitology to the various veterinary and medical courses then on offer in the department.
Allan and I attended the Parasitology Congress held in Rome in 1963, me to deliver a paper on my work with tsetse fly age determination. Unlike most other delegates, however, we eschewed comfortable flights, opting to travel overland by train. We thus travelled to London, through Belgium and down the Rhine to spend a night in Basel. The next day we continued through Switzerland, into Italy (where the train got steadily slower and hotter) to spend a second night in Pisa, before continuing our journey to Rome. We found that the Congress was not particularly well organised, with the ‘parallel sessions’ getting seriously out of synchrony so that movement from one session to another was far from easy. In the end, I think we attended the paper just before mine, the one I gave (of course!) and then the one after that – before jumping on another train down to Naples and a visit to Pompeii. We justified this considerable turpitude by the cultural education it gave us! After the Congress we made our slow progress back to Edinburgh, again by train.
I attended the Entomology Congress in London in 1964, and a meeting of the Society for Experimental Biology in Copenhagen in 1965, but generally the decade came to a quiet end with me pursuing my teaching and research activities. In the decade from 1955 to 1965 I had proceeded from being an undergraduate at King’s, to getting a Ph.D. at the London School of Hygiene and Tropical Medicine, to marriage and becoming the father of three boys, to obtaining a lectureship in Zoology at the University of Edinburgh, thus attaining my ambition of a career in Entomology. The next decade would see significant changes in the direction of my research.
The tsetse fly research led to some newspaper coverage: