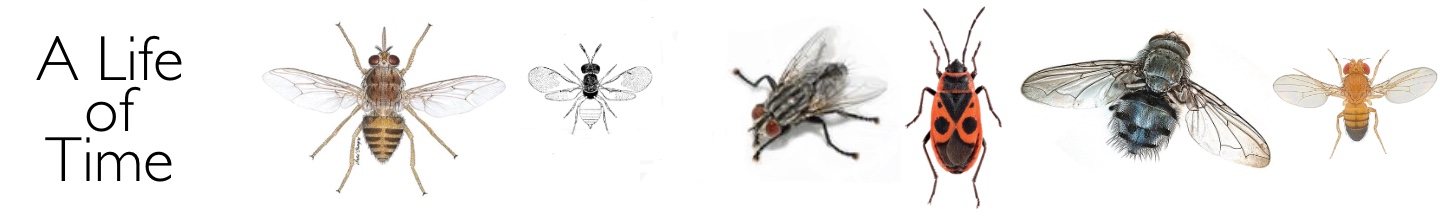
Earlier, in 1956, I had started to look for a suitable Ph.D. programme. Initially I approached Don Arthur who worked on ticks, but he had too many students and would not take me on. At that time I became very interested in parasitic Hymenoptera and their possible use as agents of biological control. After discussions with John Cloudsley-Thomson and Don Arthur I was advised to contact Professor Douglas Bertram of the Entomology Department at the London School of Hygiene and Tropical Medicine, and I went up to Malet Street for an interview. Douglas Bertram was from the ‘school’ of medical entomologists trained at Glasgow University just before the war and was working on the transmission of nematode worms by blood-sucking mites. He had a strong Scottish accent (which I later got used to!) and I am not sure that the interview went all that well – at least, I didn’t really understand what was going on. Patrick Buxton, the head of the department of Entomology had died in 1955, but his influential book “The Natural History of Tsetse Flies” had just been published. Knowing my interest in parasitic Hymenoptera, Bertram opened the book to page 426 to show me a picture of a small Eulophid wasp Syntomosphyrum glossinae that had been used on several occasions in unsuccessful attempts to control tsetse flies in eastern and southern Africa. My remit was to investigate the biology of this small wasp - its survival in different environmental conditions, its behaviour, its host range and whether it could be used successfully as an agent of biological control.
 The picture of the Eulophid parasite of tsetse fly puparia that first inspired my Ph.D. work. (From P.A.Buxton The Natural History of Tsetse Flies 1955)
The picture of the Eulophid parasite of tsetse fly puparia that first inspired my Ph.D. work. (From P.A.Buxton The Natural History of Tsetse Flies 1955)
In the October of 1956 I started at the London School on a Medical Research Council postgraduate grant which provided all my research costs and the princely sum of £400 a year. When I arrived at the Entomology department I was put in a rather large laboratory once occupied by the great insect physiologist, V.B. Wigglesworth, the discoverer of Juvenile Hormone (JH). V.B. (later Sir Vincent) Wigglesworth had joined the department in the 1930s to work with Professor Patrick Buxton who had introduced the blood-sucking hemipteran bug Rhodnius prolixus as an experimental insect. This remarkable insect imbibes a single enormous bloodmeal in each stage or instar, and each of these bloodmeals leads to either moulting (in juveniles) or to the production of eggs (in the adult female). Wigglesworth discovered the hormone (JH) in this system that essentially maintains the juvenile or larval form, and then carried this insect with him to Cambridge when he left the London School. I wish I could say that occupying this room led me to similar momentous discoveries!
Syntomosphyrum drills through the hard puparium of the tsetse fly pupal stage with its ovipositor and lays its eggs on the surface of the pupa, in the space underlying the hard ‘shell’. When the larvae hatch they feed as external parasites on the soft tissues of the pupa, killing it in the process. The wasp larvae then pupate within the host’s puparium and the emerging adult wasps finally bite through to escape. The School of Hygiene used its many contacts in Africa to obtain samples of wild collected puparia of a number of tsetse species, including Glossina morsitans, G. palpalis, G. pallidipes and G. brevipalpis. These collections came from a number of sites around Africa, including - as they were then called - Tanganyika, Southern Rhodesia, Nigeria and the Congo. These countries sounded incredibly exotic and exciting to me, and I longed to be able to visit them. Sometimes postage of these specimens caused some excitement. On one occasion the small tin containing the pupae became separated from its label, and I received an agitated ‘phone call from the post office at Mount Pleasant to say that they had some “tropical seeds” – were they mine? Needless to say, I went straight over to Mount Pleasant to claim my tropical seeds without telling them that they were tsetse fly pupae, since that might have caused (erroneously) some sort of health scare!
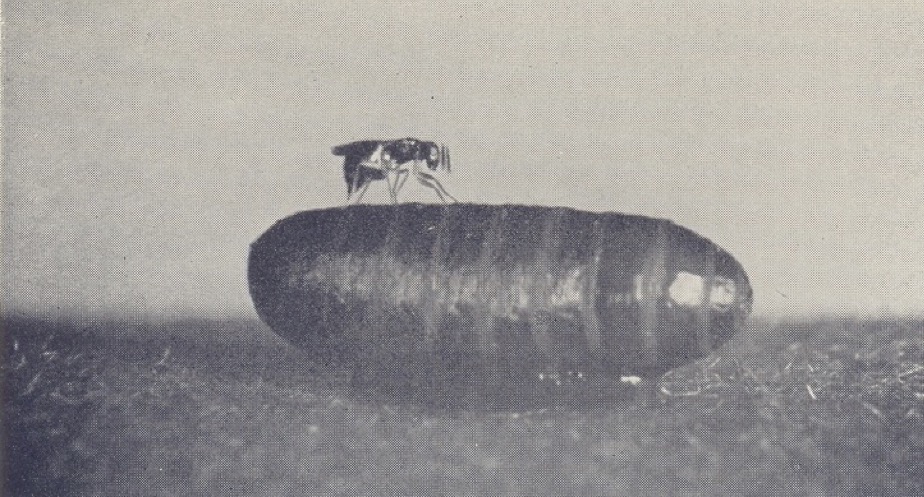

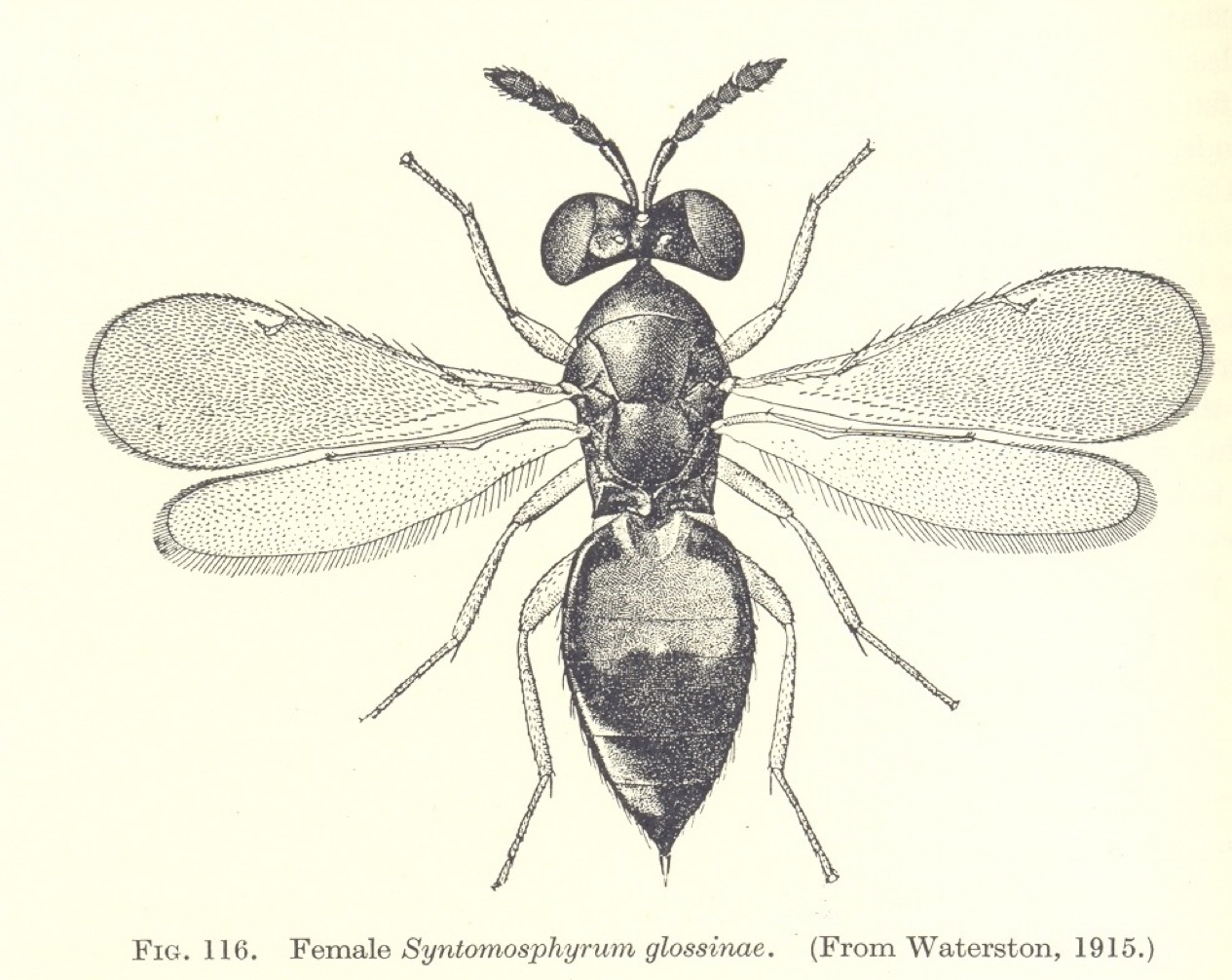
Syntomosphyrum albiclavus drilling through the puparium of a blowfly to lay its eggs (top) and the puparium opened to show mature wasp larvae
My studies on the wasps soon showed that some of the samples from East Africa were different from the others in that they had distinct white clubs at the ends of their antennae, and that this ‘white-clubbed’ form would not interbreed with the more ‘normal’ specimens. Actually, sex determination in the Hymenoptera is rather different from most other insects, in that fertilised eggs give rise to female progeny with the normal two sets of chromosomes (i.e. they are diploid) whereas unfertilised eggs only give rise to male progeny with a single set of chromosomes (i.e. they are haploid). Males are said to arise from a form of parthenogenesis called arrhenotoky. I found that reciprocal crosses between males and females of the ‘normal’ and the ‘white-clubbed’ strains of wasps only gave rise to male progeny, thus indicating that fertilisation had not occurred, whereas within-strain crosses produced both males and females. This was evidence of a reproductive barrier between the ‘white-clubbed’ and normal forms of Syntomosphyrum and therefore of their separate specific status. The ‘white-clubbed’ form was subsequently described and named as Syntomosphyrum albiclavus by G. J. Kerrich at the Natural History Museum.
Physiological and behavioural experiments using Syntomosphyrum wasps showed that their survival was severely curtailed by low relative humidity, and that they had a very wide host range. I found that they were capable of parasitising the puparia of every species of fly that I cared to offer, apparently adjusting the number of eggs laid according to the size of the host. Thus several hundred wasps could be reared from large puparia such as the flesh fly Sarcophaga argyrostoma, about 30 to 40 from blow fly puparia such as Lucilia sericata and Calliphora vicina, and usually only one from the very small puparium of the fruit fly, Drosophila melanogaster. They were even capable of investigating the egg capsules or oothecae of cockroaches, perhaps ‘thinking’ that they were ‘puparia’ but were never found to lay eggs within them. The wasps, therefore, showed a rather wide host range, and could not be regarded merely as parasites of Glossina. They were also incapable of burrowing into the soil to reach puparia and could only gain access to those potential hosts that were exposed or located in cracks. These results suggested why Syntomosphyrum wasps had failed in the various attempts to use them as agents of biological control: They were by no means host specific (to Glossina), could only reach a small proportion of their potential hosts, and – unlike an exotic parasite – had presumably achieved some sort of natural ‘balance’ between host and parasite which was difficult to upset, even by a massive release of laboratory-bred wasps.
In the course of my Ph.D. work, however, I also studied the pteromalid wasp Nasonia (=Mormoniella) vitripennis which also parasitises the puparia of ‘higher’ flies, obtaining the original colony of wasps from Dr George Salt of Cambridge. This wasp, like Syntomosphyrum, drills through the hard puparium of its host with its ovipositor and lays its eggs on the surface of the soft pupa beneath. I obtained my strain of Nasonia in the autumn of 1956, and what followed was one of the two truly serendipitous discoveries of my entomological career (the other concerning tsetse fly ovaries; see below). The Nasonia culture was raised either in the laboratory or in the departmental insectaries on the departmental roof and, quite suddenly, breeding ceased! Upon investigation, it turned out that all the larvae of Nasonia had entered diapause, becoming dormant for the winter, presumably in response to the shortening days of autumn. There was, of course, nothing really new in this, but I had discovered – for myself – photoperiodic induction of diapause. This discovery laid the foundation of a large part of my subsequent scientific career, as we will see later.
My room-mate at the London School, Willie Samarawickrema from Ceylon (now Sri Lanka) worked for his Ph.D. on mosquitoes with Brian Laurence. Using Mansonia mosquitoes he investigated a technique for age determination that had been recently described by Soviet entomologists using age changes in the ovaries. This technique needs some explanation here because it also formed the basis of much of my own later work on tsetse flies. Insect vectors, especially those transmitting protozoan parasites with rather complicated periods of development within the insect host, require a period of time between picking up the pathogen at one blood-meal and transmitting it on a later occasion: the intervening time, of course, corresponds to the time the pathogen takes within the vector to reach its infective stage. It follows, therefore, that older mosquitoes are considerably more ‘dangerous’ as vectors than younger mosquitoes; indeed an infected mosquito may take a series of blood-meals without passing on the parasite before it becomes infective. Consequently, methods to determine the age of wild-caught mosquitoes could be of utmost importance in establishing the epidemiological ‘importance’ of a group of potential vectors.
Each ovary of a mosquito contains nearly 100 egg tubes or ovarioles. Egg primordia arise from the germarium at the top of each ovariole and pass down the tube as developing oocytes. After maturation, the fully developed eggs pass out of the ovariole to be deposited in a suitable medium, in the case of mosquitoes on the surface of water. Only female mosquitoes suck blood, the protein from this source being necessary in most cases for egg production. It has long been known that female mosquitoes produce a single batch of eggs after each blood-meal, the strict relationship between blood feeding and egg production being known as ‘gonotrophic concordance’. It follows, therefore, that a mosquito that has produced, say, six batches of eggs has obtained six blood-meals in her life. A priori, such a close relationship between feeding and egg production opens the possibility of determining the reproductive and feeding history of wild-caught mosquitoes.
The principal player in the field of age determination was a Russian entomologist, Dr T.S. Detinova from the Martinovsky Institute of Medical Parasitology and Tropical Medicine in Moscow who developed the original ideas of W.N. Beklemishev and V.P. Polovodova. These workers described a quite remarkable phenomenon hitherto unrecognised in the West. They observed that a large ‘sac’ containing some cellular debris was left in the ovariole after the mature egg had been expelled. Over the next few days this large sac shrunk (but never disappeared) to leave a small ‘follicular relic’ that marked the position of the egg and, of course, was a morphological record of the occurrence of the earlier oviposition. After the next blood-meal, the second egg in that ovariole developed and was then expelled to give an expanded sac. Then a remarkable thing happened: on retraction, two - not one - follicular relics remained in the ovariole, denoting the past occurrence of two ovipositions from that particular ovariole. Moreover, after three successive blood-meals and ovipositions, three such relics remained in the ovariole, and after six blood-meals and ovipositions there were six, and so on. Careful dissection of the ovaries of wild-caught mosquitoes could, therefore, reveal with a degree of certainty how many egg (batches) had been produced, how many blood-meals had been taken, and – get this – the number of contacts between mosquito and host during which a pathogen such as the malaria parasite could be picked up or passed on. This very elegant technique was, in short, a veritable gift for the quantitative epidemiologist! Dr Detinova came to the London School of Hygiene in 1959 to give a series of lectures sponsored by the World Health Organization (WHO Monograph Series No. 47, 1962) which I could not attend, but I was determined to apply a similar technique to that other group of important insect vectors, the tsetse flies. This had to wait until I was established at Edinburgh University (see below).
So, two events occurring during my study for a Ph.D. at the London School set the scene for my future research activities. One, age determination in insect vectors, represented the past because it left a permanent record of an insect’s reproductive physiology in her ovaries, and the second, photoperiodic induction of insect diapause (in Nasonia, and then in other insects) represented the future because it concerned the way that insects prepare themselves both physiologically and behaviourally for the impending winter. These themes will be developed as this account proceeds.

Jean on our wedding day, April 11th 1959
Yet another thing happened to me at the London School of Hygiene – I met my future wife and life-long soul-mate. Jean Doughty had arrived at the School earlier in 1956 to be secretary to Dr Kingsley Sanders and his colleagues at the MRC Virus Research Group. At first I could only admire her from a distance as she always seemed to be surrounded by a group of men! By the November of 1957, however, I had plucked up enough courage to ask her to a dance and treasure hunt at King’s College. Our lunchtimes were either spent in the British Museum or on mad shopping expeditions. Kingsley and his colleagues would frequently ask Jean to go out and buy tobacco for them (they were avid smokers of Gauloises!); she would ‘phone me up and we would go together, often spending rather too much time in local coffee bars. Within six weeks we were officially engaged and I had been taken down to Sittingbourne to be introduced to her parents. We were to be married in April 1959, but only after a six-month separation after I transferred to Edinburgh.
It was during the summer of 1958 that I learnt of a possible job at the Zoology Department, University of Edinburgh, to teach medical and veterinary entomology. This vacancy arose because the resident entomologist, Doug Kettle, was arranging a two-year sabbatical to study midges (Culicoides) in Jamaica and urgently needed someone to take on the teaching in his absence. Although I was only two years into my Ph.D. programme Bertram thought that I would have enough material and encouraged me to apply.